Pharmaceutical Plant Disinfection Effectiveness Evaluation and Verification
- Addtime: 2025-08-01 / View: 289
During pharmaceutical production, ensuring a sterile production environment is a key step in ensuring drug quality and safety. Therefore, regular and effective disinfection of all areas and equipment within the pharmaceutical plant, along with scientific and rigorous evaluation and verification of its effectiveness, is crucial.
Pharmaceutical plants need to develop a disinfection effectiveness evaluation method tailored to their needs to ensure that disinfection measures achieve their intended objectives and provide a solid safety guarantee for pharmaceutical production.
First, clearly defining the evaluation targets is essential. This includes, but is not limited to, the air in the production workshop, surfaces (such as workbenches and walls), the interior and exterior of equipment, and personnel's hands—all areas that could impact product quality. Comprehensive coverage effectively avoids the risk of overall contamination caused by partial omissions.
Second, selecting the right disinfectant is crucial. Choose disinfectants that are highly effective, low-toxic, and easy to remove residues, tailored to the specific material and environmental characteristics. Furthermore, compatibility between disinfectants must be considered to avoid adverse reactions or reduced efficacy from mixing multiple disinfectants.
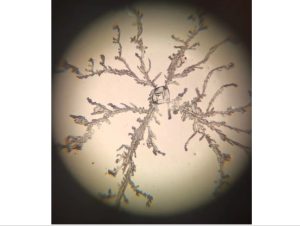
Finally, a reasonable sampling plan should be developed. Samples should be collected from specific locations within a specified timeframe after cleaning, in accordance with relevant national standards and industry guidelines. For air, the sedimentation bacteria method can be used; for surfaces, a sterile swab is used for sampling. A negative control should be included in each experiment to eliminate external interference.
Finally, the collected samples are analyzed using microbial culture techniques. Comparing the changes in data before and after the test determines whether the disinfection effectiveness has met the standards. Furthermore, rapid screening methods such as ATP bioluminescence testing can be used to assist in assessing the degree of immediate cleanliness.
Fantong Bio assists pharmaceutical companies in establishing a comprehensive disinfection effectiveness evaluation system and provides disinfection effectiveness evaluation services. This not only helps improve product quality control but also promotes continuous improvement of production processes, ultimately achieving a win-win situation for both economic and social benefits.